Four causes of hypoxemia: Abnormal ventilation/perfusion ratios as the major cause of hypoxemia.
Lisa Ebihara MD-PhD, Department of Physiology and Biophysics
Read Chapter 5 of West, Respiratory Physiology 7th ed.
Abnormally low oxygen in the blood is caused by one or more of the following: (1) Hypoventilation; (2) diffusion impairment; (3) right to left shunt (usually in the lungs, but can be in the heart); (4) abnormal ventilation/perfusion ratios.
- I. Hypoventilation: Hypoxemia develops when the pulmonary alveoli are inadequately ventilated. Clinically, the hallmark of inadequate ventilation is hypercapnia, an elevated systemic arterial PCO2 (PaCO2 > 43 mm Hg). The most common cause of hypoventilation is depression of the medullary respiratory center by drugs. The effect of hypoventilation on alveolar and system arterial PCO2 and PO2 will be illustrated an example of a man with normal lungs who takes an overdose of barbiturates which depresses his medullary respiratory center, such that alveolar ventilation is depressed by half while CO2 output remains normal. Blood gas analysis obtained with the patient breathing room air showed a PaO2 of 50 mm Hg and a PaCO2 of 80 mm Hg. When the patient was given 27% O2 to breath his PaO2 rose to ~100 mm Hg. Keep in mind the two most basic formulas in respiration: PaO2 = PiO2 - PCO2/R and PaCO2 = VCO2/VAxK
This example illustrates that:
- PAO2 is depressed approximately to the same extent that PACO2 is elevated.
- Even a 50% decrease in alveolar ventilation leaves PAO2 at 50 mmHg, a value high enough to produce 80% saturation of Hb with O2.
- The hypoxemia that is caused by hypoventilation can be remedied by administering air that is only slightly enriched with O2.
- II. Impairment of diffusion: this means that equilibration does not occur between the PO2 in the pulmonary capillary blood and alveolar gas. Recall that the diffusion capacity of the lung for a gas is equal to:
Figure 1 shows the time course of the rise in PO2 as the red blood cell moves through the capillary. Under typical resting conditions, the capillary PO2 reaches that of alveolar gas when the red cell is about one-third of the way along the capillary. Even when the capillary transit time is shortened by exercise, the capillary blood equilibriates completely with alveolar air. However, in some abnormal circumstances when the diffusion properties of the lung are impaired, the blood does not reach the alveolar value by the end of the capillary. Diffusion limitation seldom causes systemic hypoxemia at rest, but may cause hypoxemia during exercise when there is less time for equilibration with alveolar gas.

Figure 1. Changes in PO2 along the pulmonary capillary. On exercise, the time available for O2 diffusion across the blood-gas barrier is reduced. A thickened alveolar wall slows the rate of diffusion. From JB West, Pulmonary Physiology-The Essentials, 7th ed.
Diseases in which diffusion impairment may contribute to hypoxemia include asbestosis, sarcoidosis and diffuse interstitial fibrosis. Impaired diffusion is also likely to develop when PAO2 is abnormally low, such as at high altitudes. Here, the impairment occurs because the ΔP for O2-diffusion is low. Also, when the diffusion pathway is thickened, hypoxemia may not develop until the patient exercises (Why?).
Some important points-
- Carbon dioxide elimination is generally thought to be unaffected by diffusion abnormalities. This is because the diffusion of CO2 is ≥20X faster than O2. Clinically, significant hypercapnia (elevations in arterial PCO2) is never caused by a diffusion defect.
- Hypoxemia can be easily corrected by breathing an enriched oxygen mixture.
- III. Shunt: Blood returning to the right heart and entering the lungs through the pulmonary artery is called mixed venous blood. If some the cardiac output bypasses functioning lung through a shunt, then arterial blood will be a mixture of normally oxygenated blood and poorly oxygenated mixed venous blood. A shunt can occur in one of two situations:
- An anatomic shunt occurs when blood bypasses the lung through an anatomic channel, such as from the right to left ventricle through a ventricular septal defect.
- A physiological shunt occurs when a portion of the cardiac output goes through the regular pulmonary vasculature without coming into any contact with alveolar air. Physiological shunting is often seen in conditions such as pulmonary edema, pneumonia, and collapse of a portion of the lung (pneumothorax).
The following example will illustrate the effect of a large pulmonary shunt caused by occlusion of the right main bronchus.

Figure 2
(circled number- PCO2)
This example illustrates that:
- Shunting of blood through unventilated alveoli produces hypoxemia. Note that the arterial O2 content is a weighted average of mixed venous and end-capillary O2 content. You can find arterial PO2 from arterial O2 content by working back through the dissociation curve, but not by averaging mixed venous and end-capillary partial pressures of O2.
- Since shunted blood contacts no air, increasing the fraction of inspired oxygen
FIO2 will not improve oxygenation (except by adding more dissolved oxygen to the normally oxygenated blood)
- PaCO2 will usually be normal or low because increased alveolar ventilation
(stimulated by hypoxemia) lowers PaCO2.
On page 59 of his text, West gives a formula by which the fraction of shunted blood can be approximated. West writes the formula as:

Wherein CC’O2 is the oxygen content of ideal end-pulmonary capillary blood based on the alveolar gas equation; CaO2 is the arterial oxygen content; CVO2 is the oxygen content of mixed venous blood. End-capillary O2 is a calculated assuming that end-capillary PO2 is in equilibrium with the PO2 in alveolar gas; the other contents are measured. This is a straightforward mixing calculation based on conservation of matter (O2); the derivation is given in West p. 48-49. When the values from the blood of a normal person are placed in the equation, it is found that no more than 5% of venous blood is shunted through the lung. This “natural shunt” is due to the addition of venous blood to the pulmonary vein from the bronchial blood supply, and the addition of coronary venous blood to the left ventricle from the Thebesian veins.
- IV. Ventilation-perfusion abnormalities
V/Q ratio determines the PO2 and PCO2 for an individual alveolar-capillary unit.

Figure 3 nicely illustrates the relationship between PAO2 and PACO2 as the V/Q ratio is changed. From JB West, Pulmonary Physiology-The Essentials, 7th ed.
V/Q imbalance is the most common cause of hypoxemia, a result of lung units with low V/Q ratios. An extreme case is easy to grasp – the left lung gets all the ventilation and no blood flow; the right lung gets no ventilation and all the blood flow. In this case, you have a big dead space and a big shunt. This will clearly result in severe hypoxemia. In less extreme cases, a portion of the lung may be relatively underventilated and another portion of the lung may be relatively overventilated. The effect of such a mismatch between ventilation and perfusion is examined more closely below.
In Figure 4, PaO2 is plotted against the arterial oxygen content. Note that the curve is nearly flat in the range of physiologic PaO2 values (above 70 mm Hg) and falls steeply below 60 mm Hg. Points representing oxygen contents from three separate alveolar-capillary units are also shown. These units have V/Q ratios of 0.1, 1.0, and 10.0. Note that the average oxygen content after all the blood is mixed (18.5 ml O2/100 ml) is lower than the oxygen content from a normal unit (19.5 ml O2/100 ml).

Figure 4. Oxygen dissociation curve: PaO2 vs. oxygen content. Oxygen content from alveolar-capillary units with V/Q ratios of 0.1,1, and 10 are, 16, 19.5, and 20 ml O2/100 ml blood. Lines are drawn for each content to its point on the dissociation curve. The average oxygen content, 18.5 ml O2/100 ml) is lower than the oxygen content from the normal unit (19.5 ml O2/100 ml).
Hyperventilation of some units does not add enough oxygen to balance out the
low oxygen content from the hypoventilated units. The result is a final oxygen content determined mainly by the low V/Q areas.
V/Q imbalance will also cause an increase in PCO2 in the poorly ventilated alveoli. Nevertheless, systemic arterial PCO2 will remain normal because the hypoxemia stimulates increased ventilation in rest of the lung. The reason why increasing ventilation can lower CaCO2 but cannot raise CaO2 has to do with the shape of the oxygen and the carbon dioxide dissociation curves (Figure 5). The carbon dioxide curve is nearly linear over the physiological range of PaCO2’s and its slope is much steeper than the slope of the oxygen dissociation curve. Thus a reduction in alveolar PCO2 and corresponding increase in alveolar PO2 due to hyperventilation cause a large decrease in the carbon dioxide content but only a small increase in oxygen content.

Figure 5. V/Q imbalance and the dissociation curves for carbon dioxide and oxygen. v/Q represents low V/Q units and V/Q represents high V/Q units.
The hyperventilation in the remainder of the lung increases the physiological dead space (VDAS) of the lung. The increase VDAS represents “wasted ventilation” because it is not able to raise the PO2 of the pulmonary venous blood to normal. In most lung diseases in which ventilation is sufficiently impaired to produce hypoxemia, VDAS is increased.
Hypoxic vasoconstriction (covered in an earlier lecture) will decrease blood flow to the pulmonary capillaries of the poorly ventilated alveoli. This will diminish the extent of the V/Q mismatch, and thus diminish the extent of hypoxemia.
An example of a condition in which the primary disorder is a high V/Q ratio is pulmonary embolism as shown in the figure below.

Figure 6
Important facts to understand regarding pulmonary embolism are:
- Theoretically, hypoxemia should not develop in response to occlusion of an artery, and occlusion of a small branch does not produce hypoxemia. Clinically, occlusion of a pulmonary artery or a major branch of it often does result in hypoxemia. The reasons for the hypoxemia are several. First, sudden loss of a large portion of the pulmonary circulation greatly increases resistance to flow. The resulting increase in pulmonary artery pressure may lead to interstitial edema. The increased pulmonary arterial pressure and the interstitial edema increase the width of the diffusion pathway. Also, blood flows through the pulmonary capillaries more rapidly, leaving less time for diffusion. The edema also decreases the compliance of the lung thereby increasing the work of breathing. Clinically, dyspnea (difficult breathing) often results from a large embolism. Second, the occluded region probably releases vasoconstrictor substances (serotonin has been suggested), which further increase resistance to blood flow and the work that the right heart must perform. Third, pulmonary shunts develop in some patients with pulmonary embolisms. West (in his pulmonary pathophysiology text) suggests that A-V anastomoses always exist in the lung, but they are normally closed. The high pulmonary arterial pressures in patients with large pulmonary embolisms cause these anastomoses to open.
- In a patient with a large pulmonary embolism, the end-expired PCO2 will be considerably lower than the arterial PCO2. These two values are normally about equal, because the end-expired PCO2 represents alveolar PCO2. In pulmonary embolism, many of the alveoli are unperfused, and contribute no CO2 to the expired air. VDAS is abnormally large.
- V. Differences in VA/Q ratios in the normal lung: Base versus Apex
- For mechanical reasons that will be described in subsequent lectures, in an upright subject. Q and VA are greater at the base of the lung than in the apex. While the apex is poorly ventilated and poorly perfused, its perfusion is even poorer than its ventilation. The effect of this is a very high VA/Q ratio at the apex. This relationship is illustrated in Figure 5.8 amd 5.9 from JB West, Pulmonary Physiology-The Essentials, 7th ed.
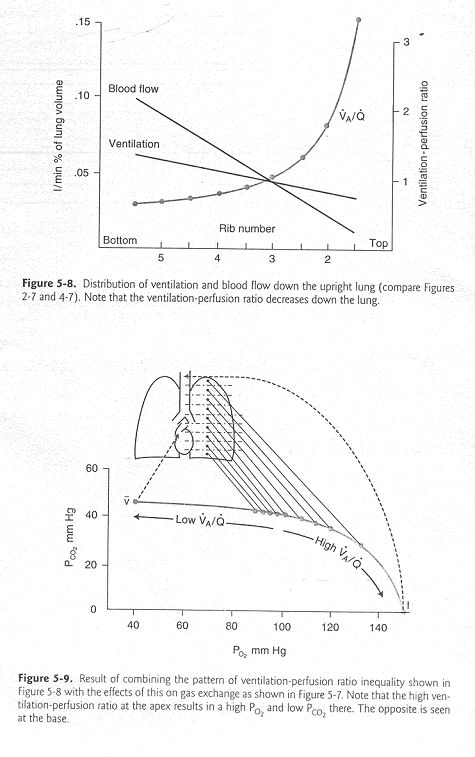
The effect of the high VA/Q ratio at the apex (and the lower ratio at the base) on the alveolar PO2, PCO2 and pulmonary capillary pH is illustrated in Figure 5.10 from West. Notice that in spite of the lower VA/Q ratio at the base, (1) Hb in basal pulmonary capillaries is 95% saturated with O2; (2) total O2 uptake and CO2 egress is far greater at the base of the lung than at the apex.

- VI. Distribution of ventilation:perfusion ratios (a specialty of West): By using a technique that we need not be concerned with, it is possible to determine how much blood flow (Q) and how much alveolar ventilation (VA) is distributed to lung regions with a particular VA/Q ratio. Not surprisingly, in people with normal pulmonary systems, most VA and most Q goes to lung regions with a VA/Q ratio of about 1 (Figure 5.13 from West). In a patient (Figure 5.14 from West) with obstructive lung disease (chronic bronchitis and emphysema), considerable blood flows to very poorly ventilated regions (VA/Q=1), and at the same time, considerable ventilation goes to lung regions that are poorly perfused (VA/Q=1.0-10). This dramatically illustrates VA/Q mismatch which West maintains is the most common cause of hypoxemia.


- VII.Measurement of VA/Q mismatch:
- The extent of VA/Q mismatch can be estimated by measuring VD (physiologic dead air space) using a formula developed on page 18-19 of West:
VD/VT is the ratio of physiologic dead air space to tidal volume.
PaCO2 is the measured arterial PCO2. Patients with VA/Q almost always have uneven ventilation causing the alveoli to empty unevenly. For this reason, end-expired air is not a reliable measure of PaCO2. Arterial PCO2 (PaCO2) is the best estimate of PaCO2 obtainable.
PCO2(mixed exp) is the PCO2 of mixed expired air which, of course, contains a mixture of physiologic DAS and alveolar air.
VD/VT is 0.2-0.35 in normal nonexercising subjects. In patients with VA/Q mismatch, it is greater than 0.35 and can even be 0.5, i.e. half the ventilation is “wasted”.
- Another way of estimating VA/Q mismatch is to measure systemic arterial PO2 and then compare this value with the ideal alveolar PO2. In other words, determine the alveolar-arterial O2 difference. The ideal alveolar PO2 is estimated from the alveolar gas equation which was developed in an earlier lecture. In a subject with a normal pulmonary system, the alveolar-arterial O2 difference is only about 5 mm Hg. In a subject with VA/Q mismatch, the difference can be much larger (40 mm Hg). Of course, an alveolar-arterial difference will also be seen in hypoxemia caused by pulmonary or intracardiac shunt. Shunt can be distinguished from VA/Q mismatch because the latter’s hypoxemia is correctable by breathing 100% O2.
VENTILATION-PERFUSION MISMATCH IN RESPIRATORY DISEASES
Ventilation-perfusion abnormalities occur in many pulmonary diseases. These diseases include obstructive, restrictive and vascular pulmonary diseases. The main pathology and clinical problems observed in several different pulmonary diseases are discussed below:
OBSTRUCTIVE DISEASES
Chronic Obstructive Pulmonary Diseases (COPD)
- 1. Pure emphysema (COPD type A)
Pathology: Areas of greatly enlarged air spaces with disruption of alveolar walls.
VDAS (physiological dead space). Large increase due to alveoli with high VA/Q ratio. Enlarged air spaces have much reduced blood perfusion.
Shunt: Minimal. Absence or minimal presence of unventilated alveoli.
Alveoli with low VA/Q ratio: Small increase, due to some shift in blood flow from capillary deprived(emphysematous) air spaces to normal alveoli and consequential small drop in VA/Q in the latter.
Diffusion impairment: None
Hypoxemia: Mild (PaO2 = ~80) due to low VA/Q regions (see above). (A-a)PO2 = 10-15 (air breathing).
Hypercapnia: None (PaCO2 = ~ 40). As long as VE is increased to compensate for increase in physiological dead space, PaCO2 is normal.
Acid/base problems: None as long as PaCO2 is normal.
Tissue oxygenation: Normal.
- 2. Pure chronic bronchitis (COPD type B)
Pathology: Abundant mucous secretions in bronchial tree and narrowing of small airways due to inflammation and wall edema.
VDAS: Small to moderate increase in alveolar dead space ventilation due to small areas with high VA/Q ratio.
Shunt: Minimal. Absence or minimal presence of unventilated alveoli.
Alveoli with low VA/Q ratio: Large increase, due to excessive blood flow to poorly ventilated alveoli. Some compensation is provided by hypoxic vasoconstriction.
Diffusion impair.: None.
Hypoxemia: Significant to severe (PaO2 = 40-70) due to low VA/Q regions. (A-a)PO2 = 20-50 (air breathing).
Hypercapnia: Moderate (PaCO2 = ~50). Increased airway resistance due to bronchial obstruction and narrowing of small airways results in chronic hypoventilation.
Acid/base problems: Minimal to moderate acidosis, in spite of increased PaCO2, due to compensatory retention of bicarbonate by the kidneys.
Tissue oxygenation: Normal as long as severe hypoxemia is compensated by increase in
RESTRICTIVE DISEASES
- 1. Interstitial pulmonary fibrosis
Pathology: Thickening of alveolar wall due to interstitial infiltration with lymphocytes, plasma cells and collagen fibers.
VDAS: None or minimal.
Shunt: Minimal. Absence or minimal presence of unventilated alveoli
Alveoli with low VA/Q ratio: Moderate amount due to some reduction of ventilation in alveoli with relatively normal blood flow.
Diffusion impair.: Minimal at rest, in spite of several fold increase in the alveolar gas-to-blood distance, due to the great diffusion reserve available (normally blood gases equilibrate with alveolar gases in the first third of the time spent in an alveolar capillary), but significant with exercise, due to increased velocity of blood circulation (reduced time spent in alveolar capillary).
Hypoxemia: Moderate at rest (PaO2 = ~80) due to low VA/Q regions (see above), but significant with exercise due to diffusion impairment added to effect of low VA/Q regions.
Hypercapnia: Absent. Usually there is mild hypocapnia (PaCO2 = 30-40) probably due to stimulation of receptors in the lung (stretching of airways due to increased lung recoil).
Acid/base problems: Mild alkalosis, as long as the hypocapnia is not compensated by a drop in bicarbonate.
Tissue oxygenation: Normal as long as hypoxemia is compensated by increased Q.
VASCULAR DISEASES
- 1. Pulmonary edema
Pathology: Accumulation of fluid in alveolar air space and in alveolar wall, most often caused by left ventricular failure resulting in increased hydrostatic pressure in pulmonary capillaries and pulmonary veins.
VDAS: None or minimal.
Shunt: Significant, due to unventilated alveoli because of intra-alveolar fluid.
Alveoli with low VA/Q ratio: Moderate amount, due to partial airway obstruction by fluid and regional VA/Q drop.
Diffusion impair.: Minimal at rest, in spite of interstitial fluid, due to the great diffusion reserve available (see above).
Hypoxemia: Moderate (PaO2 = 70-80) due to shunted regions (VA/Q = 0)
and regions with low VA/Q ratio.
Hypercapnia: Absent of mild hypocapnia due to stimulation of receptors in the lungs, and to hypoxemia if PaO2 drops below ~70 mm Hg.
Acid/base problems: None or mild alkalosis, as long as the hypocapnia is not compensated by a drop in bicarbonate.
Tissue oxygenation: Normal as long as hypoxemia is compensated by increased Q.
- 2. Pulmonary embolism
Pathology: Acute occlusion of branches of the pulmonary artery most often caused by thrombi that had come loose from the walls of deep veins of lower extremities, pelvic regions, inferior vena cava or right heart.
VDAS: Significantly increased, depending on the size of the affected area, due to drastic reduction of regional alveolar perfusion (large regional increase in VA/Q).
Shunt: Significant, due to blood flow through unventilated alveoli in infracted regions (hemorrhagic atelectasis) and opening of A-V anastmoses.
Alveoli with low VA/Q ratio: Significant amount, due to redistribution of blood flow from obstructed arteries to normal lung regions. This results in excessive perfusion of normally or poorly ventilated alveoli with sizable drop in regional VA/Q.
Diffusion impair.: Minimal to moderate, due to increased regional blood flow velocity resulting in reduced transit time.
Hypoxemia: Moderate (PaO2= 70-80) due to shunted regions (VA/Q = 0)
Hypercapnia: Absent or mild hypocapnia due to the effect of hypoxemia on chemoreceptors, if PaO2 drops below ~70 mm Hg.
Acid/base problems: None or mild alkalosis.
Tissue oxygenation: Normal as long as hypoxemia is compensated with increased Q.