Circulation Through Individual Tissue Beds (Chapter 25)
Objectives
- Explain the factors that affect coronary blood flow
- Describe the regulation of cutaneous blood flow
- Explain the factors affecting muscle blood flow at rest and exercise
- Describe the regulation of cerebral blood flow
- Explain the regulation of the splanchnic circulation
CORONARY BLOOD FLOW
Each tissue bed in the body has a specialized vascular supply to support its function. While all vascular beds follow the general rules of flow and pressure control previously described, each has special adaptations.
- Coronary Circulation: The control of blood flow to the heart muscle is similar to that of most muscles; flow increases in response to local metabolites produced by the working muscle. While flow is proportional to the rate of work, it is impeded mechanically during contraction.
Coronary blood flow is about 250 ml/min in a normal heart at rest. Approximately 80% of the flow supplies the left heart and the remainder goes to the right. Coronary blood flow accounts for 5 % of cardiac output
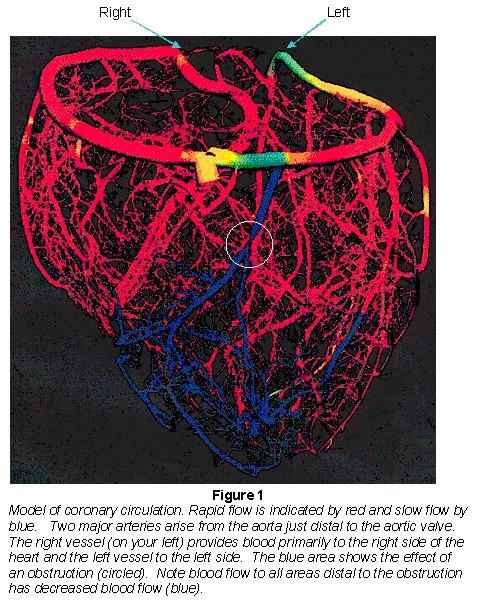
The major coronary arteries arise from the aorta immediately distal to the aortic valve and provide virtually all the arterial blood supply (Fig. 1). (A very small portion of blood flow to the myocardium comes is through the arterioluminal arteries that are supplied with blood directly from the ventricular lumen.)
Venous drainage of the myocardium: Coronary capillary blood flows via venules to veins that reach the epicardial surface of the heart and parallel the arterial supply back to the Coronary Sinus. Most of the left ventricular capillary blood reaches the right atrium by way of the coronary sinus, and a smaller portion, the blood from the right ventricle capillaries, by way of the anterior cardiac vein. A very small amount of blood reaches the cardiac ventricles directly, through the thebesian veins (Fig. 2).
(Reprinted from Circulatory Physiology-the essentials 2nd ed.,(1984) by j.j.smith & J.P. Kampine, figure 11.8, page 189, Lippincott, Williams & Wilkins.)

- Factors that influence coronary blood flow:
- Aortic pressure provides the driving force for blood flow through the coronary arteries. During diastole flow is directly proportional to aortic pressure. Low aortic pressure may limit coronary blood flow in severe hypotension (e.g. shock), and the increase in mean aortic pressure during exercise contributes to increased coronary blood flow. For reasons discussed below, aortic pressure during diastole is most closely correlated with coronary blood flow.
- Extravascular compression (squeezing) of the myocardial vessels occurs during cardiac systole (Fig. 3). Like other muscles, contraction of the ventricular muscle compresses the blood vessels within its wall. Compression reduces vascular diameter, increases vascular resistance, and decreases flow. During isovolumic contraction of the left ventricle, extravascular compression is so great that flow in the left coronary artery may reverse momentarily. During diastole the myocardium is relaxed, no vascular compression occurs and blood flow thru the myocardium is at its greatest.
Extravascular compression is a particular problem with sustained hypertension. The elevated afterload on the left ventricle increases ventricular work and O2 demand while also increasing extravascular compression. The combination increases the need for blood flow and restricts it as well.

(Reprinted from Principles of Physiology 3rd ed., (2000) by R.M. Berne & M.N. Levy, page 280 with permission from Elsevier.)
Pressure within the wall of the left ventricle (myocardial tissue pressure) is greater near the inside (endocardial surface) than near the outside (epicardial) surface; thus extravascular compression is more significant near the endocardium. For this reason, in conditions with reduced blood flow to the heart (coronary occlusion or hypotension) damage to the inner (endocardial) wall of the ventricle is more probable than to the outer (epicardial) wall.
Because myocardial pressure during contraction is not nearly so great in the right ventricle, extravascular compression does not significantly alter coronary flow to the right ventricle and flow in the right coronary artery is greater during systole than diastole because of the greater driving pressure during systole.
- Sympathetic Effects on Coronary Blood Flow
Stimulation of the sympathetic nerves to the heart increases coronary blood flow indirectly. (If the inotropic and chronotropic effects of sympathetic stimulation are blocked with a β-adrenergic blocker, sympathetic stimulation actually decreases blood flow, suggesting that α-adrenergic vasoconstriction of the coronary vessels has occurred.) In normal circumstances, sympathetic stimulation increases coronary blood flow by increasing heart rate and contractility, which, in turn, increases cardiac work and myocardial O2 consumption. The resulting decrease in tissue PO2 increases coronary blood flow by metabolic regulation.
- Coronary Blood Flow by is Controlled by Metabolic Factors.
With the limitations of aortic pressure and extravascular compression coronary blood flow is directly related to level of cardiac work and O2 demand of the myocardium, even in a denervated heart (Fig 4). A number of metabolites are implicated in the metabolic mechanism by which O2 demand triggers coronary vasodilatation including: lactic acid, H+ (decreased pH), adenosine endothelial-derived releasing factor (NO) and K+.

- Some Clinical Aspects of Coronary Blood Flow
Slow, progressive ischemia (causing hypoxia) of myocardial tissue stimulates the growth of collateral vessels allowing more blood (O2) to reach the ischemic area.
Ischemia of cardiac tissue gives rise to chest pain (angina pectoris). Angina pectoris is often relieved by the administration of nitrites (e.g., nitroglycerin or amyl nitrite). The nitrites, which give rise to NO, act as general systemic vasodilators and especially dilating coronary arterioles, decreasing the afterload against which the left ventricle works. This, in turn, decreases myocardial O2 demand, relieves the myocardial hypoxia and the angina.
- Cardiac O2 consumption is a function of cardiac work:
Myocardial O2 consumption of the heart in the non-exercising individual is relatively high (8-10 ml/min/100g of heart) reflecting the fact that even "at rest" the heart is working. In the non-exercising person, venous blood leaving the heart is only 25 % saturated with O2; thus only a small part of the increased demand for O2 during exercise can be met by more complete extraction of O2. Nevertheless, O2 consumption increases as much as five-fold during exercise. Therefore, the increased delivery of O2 during exercise can only be met by increasing coronary blood flow.
In physical terms, work = pressure x volume. For the heart, aortic pressure represents the pressure against which the heart ejects the stroke volume. The work the heart performs is the same whether the heart moves a stroke volume of 120 ml against a mean aortic pressure of 90 mm Hg, or a stroke volume of 60 ml against a mean aortic pressure of 180 mm Hg. However, the O2 requirement for generating pressure (pressure work) is greater than volume work. In other words, elevating aortic pressure increases O2 demand more than increasing stroke volume.
Since the right and left hearts have the same cardiac outputs over time, but the pulmonary artery systolic pressure is only about one-fifth of aortic systolic pressure, the right heart does only about one-fifth as much pressure work as the left. Therefore, the right heart requires only one-fifth of the O2 and blood supply.
- Estimating Cardiac Work and Oxygen Consumption
Estimating myocardial O2 consumption or demand (primarily left ventricular) must take into consideration both the pressure work and volume work done by the heart. As noted above the pressure work accounts for the majority (perhaps 98%) of the cardiac work at normal cardiac outputs. An index of cardiac work is the tension-time index.
tension-time index = mean pressure during systole x heart rate x duration of systole
- Effect of Ventricular Diameter on Cardiac Work
The pressure work of the heart is increased by cardiac dilation, a common adaptation to cardiac failure. In its early stages dilation of the heart provides an advantage in terms of increasing resting length of the muscle (increased preload) that helps maintain stroke volume. However, with severe dilation of the heart, its radius increases. According to the Law of LaPlace, the tension in the ventricular wall required to generate a given luminal pressure increases with the radius of the heart. Therefore, the tension against which the heart must contract increases with dilation. In turn, the O2 requirement for the increased work also increases and demands greater blood flow.
- Substrate Utilization by the Heart
To generate the energy required for contraction, the heart metabolizes whatever fuel is most readily available. If a person is in the postabsorptive state (after eating and not exercising), and blood glucose concentrations are normal, 40% of the heart's energy is derived by metabolizing carbohydrate (mostly glucose and lactate), and 60% from non-carbohydrate (mostly fatty acids) sources. As the interval after eating lengthens, the proportion of energy obtained from carbohydrate decreases, and that obtained from fatty acid metabolism increases. Also, prolonged fasting and/or strenuous exercise increases the plasma concentration of ketone bodies, which are also utilized by the heart. Ketone bodies are also produced in large quantities in diabetic acidosis under which circumstances they are metabolized by the heart.
The heart produces lactic acid from glycogen breakdown whenever inadequate O2 is delivered to the myocardium to support anaerobic carbohydrate metabolism. But, during these periods of inadequate O2 supply, the heart cannot further metabolize lactic acid (or ketone bodies) for additional energy. Thus the hypoxic (ischemic) heart is dependent on the ATP generated from anaerobic metabolism for energy.
BLOOD FLOW TO THE SKIN
- Anatomy of Skin Vasculature
Blood that enters skin arterioles can either pass through skin capillaries or arteriovenous anastomoses that directly connect arteries to veins, bypassing capillaries. Whichever pathway is followed, blood flows into venules and extensive venous plexuses that are close to the surface of the skin. Increasing blood flow through either skin capillaries or arteriovenous anastomoses increases the volume of blood flow close to the skin surface.
Normal skin blood flow rate under neutral temperature conditions ranges from 3 to 5 ml/100g of skin, but may increase to 150 ml/100g in a hot atmosphere. More than any other tissue, blood flow to the skin is under the control of the autonomic nervous system. Adrenergic vasoconstrictor fibers that innervate the smooth muscle of arterioles and arteriovenous anastomoses elicit vasoconstriction thru α-1 receptor stimulation and decrease flow of blood to the skin. Blushing of the face, ears and chest during embarrassment testifies to the high degree of neural control of blood flow to the skin. Raynaud's Syndrome is caused by vasospasm of skin blood vessels. Exposure to cold triggers this painful reduction of blood flow, especially to the hands.
- Significance of Vascular Mechanisms to Temperature Regulation
A major function of the skin is to regulate body temperature by either facilitating or reducing heat transfer from the skin surface. Skin is otherwise a slowly metabolizing tissue, requiring minimal blood flow for metabolism. Blood flow to the skin increases when hypothalamic temperature increases, and decreases when hypothalamic or skin temperature decreases. These changes in flow to the skin are mediated by changes in α-adrenergically mediated tone of the skin blood vessels. Increasing blood flow to the skin facilitates loss of heat from the body to the environment. Reducing blood flow to the skin further insulates the body from heat loss.
Arteries and veins that enter and exit the extremities generally run close to one another. This allows the heat of arterial blood to be conducted (by counter-current exchange) to the cold blood returning from the extremity, thus conserving body heat.
While exposure of the extremities to severe cold at first produces strong vasoconstriction, as the hands or feet reach near freezing temperatures, vasodilatation (mediated by relaxation of arteriovenous anastomoses) occurs. While this protects the hands and the feet from freezing, it does so at the expense of lowering core body temperature. The primacy of neural control over local control is demonstrated by the occurrence of frostbite, cold-induced tissue damage.
- Other Vascular Phenomena in the Skin
When activated, sympathetic cholinergic fibers elicit sweating. Sweat contains bradykinin, which serves as a local vasodilator.
Injured skin releases vasodilators that modify local blood flow to the injured region. The vasodilators, which may be histamine, ATP, substance P, bradykinin, or other substances, are responsible for the red lines, white lines, and wheals (the "triple response") that appear on scratched skin.
BLOOD FLOW TO SKELETAL MUSCLE
Blood flow through resting skeletal muscle is low (4 to 6 ml/100g), commensurate with the low metabolic needs of the tissue at rest. Yet, since skeletal muscle represents such a large portion of body weight, this tissue receives 20-25% of cardiac output at rest. In resting skeletal muscle as many as 90% of capillaries are closed. High vascular smooth muscle tone is maintained by strong sympathetic input. However, blood flow to exercising muscle increases as much as twenty-fold during maximal exercise even though sympathetic input to the working muscle increases. This increased flow occurs because the local metabolic control mechanisms override the increased sympathetic input. Under maximal exercise skeletal muscle may receive 90% of the cardiac output.
Exercise usually involves alternating contraction and relaxation of muscle groups. The capillaries and veins are compressed during the contraction phase, facilitating venous return of blood to the heart.
β-2 receptors in arterioles of skeletal muscle beds do not receive neural innervations, but respond to circulating β-2 agonists (e.g. epinephrine) with dilation. Cholinergic receptors on the same arteriolar smooth muscle may be innervated by sympathetic cholinergic fibers that also produce vasodilatation.
BLOOD FLOW TO THE CENTRAL NERVOUS SYSTEM
The cerebral vessels receive nearly 15% of cardiac output although the CNS only accounts for 2% of body weight.
- Unique aspects of Cerebral Blood Flow
- Consistent, adequate flow of blood to the brain is required because the brain tolerates ischemia poorly. Total interruption of blood flow to the brain for 5 seconds produces unconsciousness. Only a few minutes of ischemia results in permanent brain damage. Brain has almost no energy reserves. Local brain blood flow is under metabolic control.
Ischemia of the brain elicits (1) increased local production of vasodilators (possibly adenosine, lactic acid, H+, CO2, and K+) that reduce cerebral arteriolar resistance, thus helping to re-establish adequate cerebral blood flow; (2) constriction of the systemic vasculature of the skin, splanchnic bed, and muscles, thus diverting blood to the brain. Simultaneously, the cardiovascular center, through elevated sympathetic output, stimulates an increase in the cardiac contractility and peripheral resistance to increase arterial pressure. Collectively, these events protect the brain from underperfusion. The combination of hypertension and bradycardia (baroreceptor response to hypertension) seen in response to increased intracranial pressure following a head injury are referred to as Cushing's reflex.
- Because the brain is encased in a rigid structure, blood flow into the brain cannot exceed flow out. This is quite unlike skin, muscle, or splanchnic bed in which the blood volume contained in the organ can change considerably.
Even though total blood flow to the brain is relatively constant, some sharing of blood flow within the brain does occur. For example, shining flashes of light on the retina increased blood flow to, and metabolism within, the visual cortex occurs. Consequently, flow is decreased in other brain areas. Increases in flow are also believed to be mediated by higher local concentrations of adenosine, lactic acid, H+, CO2 , and K+.
- The central nervous system is highly sensitive to changes in extracellular environment. Interstitial concentrations of ions and other molecules must be carefully controlled. Capillary walls in the central nervous system are much less permeable than capillaries elsewhere, in part related to the continuous endothelium and tight junctions between endothelial cells. The relative isolation of CNS interstitial fluid from plasma is described as the blood-brain barrier.
- Arteries leading to the brain show myogenic autoregulation (Fig. 5).

(Reprinted from Circulatory Physiology-the essentials 2nd ed.,(1984) by J.J. smith & J.P. Kampine, Figure 11.5, page 148, Lippincott, Williams & Wilkins.)
BLOOD FLOW TO THE SPLANCHNIC BED
The splanchnic bed receives 25% of the cardiac output. The splanchnic circulation supplies blood via the celiac, superior mesenteric, and inferior mesenteric arteries to the gastrointestinal tract, spleen, pancreas, and liver. The capillaries that supply the gastrointestinal tract and pancreas feed into the portal vein, which transports venous blood at low pressures (10 to 2 mmHg) to the sinusoids of the liver. The liver also receives direct arterial supply via the hepatic artery.

(Reprinted from Circulatory Physiology-the essentials 2nd ed.,(1984)by J.J.Smith & J.P. Kampine, Figure 11.14, page 201, Lippincott, Williams & Wilkins.)
The intestinal circulation serves to transport products of digestion that have been absorbed to the liver and the rest of the body (Fig.6). In general, the splanchnic circulation is under strong sympathetic tone that greatly reduces splanchnic blood flow. During severe exercise and during hemorrhage, blood flow to the intestine can be even further reduced by α-mediated vasoconstriction. Under these circumstances blood flow is diverted from the splanchnic bed to other tissues. Secondly, venous splanchnic blood returns to the heart and is also diverted to other tissues. In this manner, the splanchnic bed acts as a reservoir of blood.
However, during absorption gastrin and cholecystokinin, intestinal hormones secreted during digestion, increase blood flow to the intestine. Also, the products of carbohydrate and fat digestion (glucose and free fatty acids) increase blood flow to the mucosa.
A unique aspect of the intestinal circulation renders it especially susceptible to anoxia and tissue necrosis. In the intestinal mucosa an arteriole runs from the base to the tip of each villus; it then divides into capillaries that merge into venules that travel back down the villus in close juxtaposition to the arteriole. Thus blood in the capillaries and venules of the villus flows in the opposite direction as in the arteriole. This counter-current exchanger allows some O2 at the base of the villus to diffuse directly from the arteriole to the venules - never reaching the tip of the villus. The value of this arrangement is that little O2 is lost by diffusion into the intestinal lumen, a largely anaerobic environment. However, when blood flow to the intestinal mucosa is especially low (as in hemorrhagic shock), cells at the tip of the villus may become severely hypoxic, even to the point of causing necrosis. The hypoxia and necrosis increase the permeability of the intestinal mucosa to bacteria and other organisms. Especially dangerous is the entry of gram-negative bacteria (e.g. E. coli) that produce endotoxins that have severe consequences for the cardiovascular system and may produce cardiovascular shock.
The liver receives about 25% of the cardiac output. About three quarters of this is delivered via the portal vein and one quarter via the hepatic artery. Because the blood in the hepatic artery contains much more O2, the majority of the liver's O2 supply comes from the hepatic artery. Blood from the hepatic artery and the portal vein mixes in the liver sinusoids. The blood then flows peripherally between hepatic cells and collects in venules. The venules eventually coalesce into the hepatic veins that feed into the inferior vena cava.
Pressure in the liver sinusoids is only 2-3 mmHg, only slightly above that of the inferior vena cava and right atrium. Since mean pressure in the hepatic artery is about 90 mmHg and pressure in the portal vein is about 10 mmHg, most of the pressure drop in the liver is pre-sinusoidal.
With cardiac failure central venous pressure may well exceed hepatic capillary pressures. The resulting elevation of hydrostatic pressure in the hepatic interstitium causes exudation of fluid through the permeable liver capsule and into the abdominal cavity (abdominal edema or ascites).
About 15 % of the blood volume is in the liver. Stimulation of the
α-adrenergic innervations of the hepatic or the portal vessels supplying the liver results in vasoconstriction and a reduction in hepatic blood flow. When this occurs during hemorrhagic shock, it not only reduces flow to the liver, but also causes about half the blood normally in the liver to enter the general circulation. Thus the liver also serves as a blood reservoir.
Cirrhosis of the liver results when hepatic cells are replaced by fibrous tissue. One effect of these changes is to increase resistance to blood flow through the liver. Since the pressure difference from portal vein to inferior vena cava is small, small increases in resistance can markedly reduce liver blood flow. Portal venous hypertension may result, reducing intestinal blood flow and nutrient uptake, and further damaging liver function.
- Blood Flow to the Kidneys
This topic will be covered in detail in connection with renal function. However, several characteristics are noteworthy. The kidneys account for less than 1% of body weight but receive 20% of cardiac output. This is related to the significant filtration function of the kidneys in maintaining body fluid homeostasis. Renal vascular beds, like splanchnic beds, have a portal architecture, with one capillary bed following another in series. Renal blood flow can be reduced by sympathetic stimulation, but overall renal circulation is strongly autoregulated under a wide range of arterial blood pressures.
Comments (0)
You don't have permission to comment on this page.